Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concepts of s and p blocks, the fundamental divisions within the periodic table.
ii. Identify the location and characteristics of s and p block elements based on their electron configurations.
iii. Differentiate between s and p block elements based on the orbitals occupied by their valence electrons.
iv. Recognize the significance of s and p blocks in understanding periodic trends and chemical properties of elements.
v. Apply the knowledge of s and p blocks to categorize elements and predict their chemical behavior.
Introduction
The periodic table, a comprehensive arrangement of elements, is not a uniform entity. It consists of distinct blocks, each characterized by the type of orbitals occupied by the elements' valence electrons. Among these blocks, s and p blocks stand out as the most prominent and well-understood.
i. S and P Blocks: A Tale of Orbital Occupancy
The division of the periodic table into s and p blocks is based on the orbitals in which the valence electrons reside:
s Block: Elements in the s block have their valence electrons in s orbitals, the first and simplest type of orbital.
p Block: Elements in the p block have their valence electrons in p orbitals, the second type of orbital, which can accommodate up to six electrons.
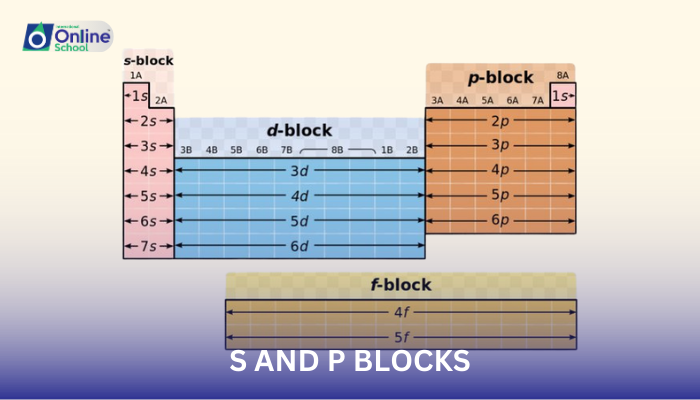
ii. Location of s and p Block Elements
s and p block elements occupy distinct regions in the periodic table:
s Block: s block elements occupy Group 1 (alkali metals) and Group 2 (alkaline earth metals).
p Block: p block elements occupy Groups 3 to 18, excluding Group 8 (noble gases).
iii. Characteristics of s and p Block Elements
s and p block elements exhibit distinct characteristics:
s Block: s block elements are generally reactive metals with low ionization energies and large atomic radii.
p Block: p block elements encompass a wide range of properties, including metals, nonmetals, and metalloids. Their properties vary depending on the number of valence electrons and the specific orbitals they occupy.
iv. Significance of s and p Blocks
s and p blocks hold immense significance in understanding chemistry:
Periodic Trends: s and p blocks showcase periodic trends in properties such as electronegativity, ionization energy, and atomic radii.
Chemical Properties: The type of s or p orbital occupied by valence electrons significantly influences an element's chemical behavior, including bonding patterns and reactivity.
Categorization of Elements: s and p blocks provide a framework for categorizing elements and predicting their similarities in properties.
s and p blocks, the foundational divisions within the periodic table, provide a valuable framework for understanding the properties and behavior of elements. By delving into the concept of s and p orbitals and their electron occupancy, we gain insights into periodic trends, chemical patterns, and the diverse nature of elements, laying the foundation for further exploration in the fascinating realm of chemistry.